You innovate,
CRISP documents
Innovating medical products is exciting and stimulating. Documenting your work is not.
This motivated us to develop CRISP, a dedicated software for documenting your product development activities.
Turn your concept into a robust design

Have questions or want to learn more about our product? Contact us today!
DualTrack, LLC
Duvall, WA 98019
info@dualtrack.llc




Innovating medical products is exciting and stimulating. Documenting your work is not.
This motivated us to develop CRISP, a dedicated software for documenting your product development activities.
Gaps in the documentation are costly...
Unverifiable data, loss of critical knowledge, poor decision making, and the need to re-track, re-search, re-do, and re-test.


Dedicated software modules capture all development activities from Day 1.
Configure, select, upload, hyperlink. CRISP takes care of the rest.
Concepts, prototypes, testing, voice of customer, root cause analysis, and more...
Everything is in one place.
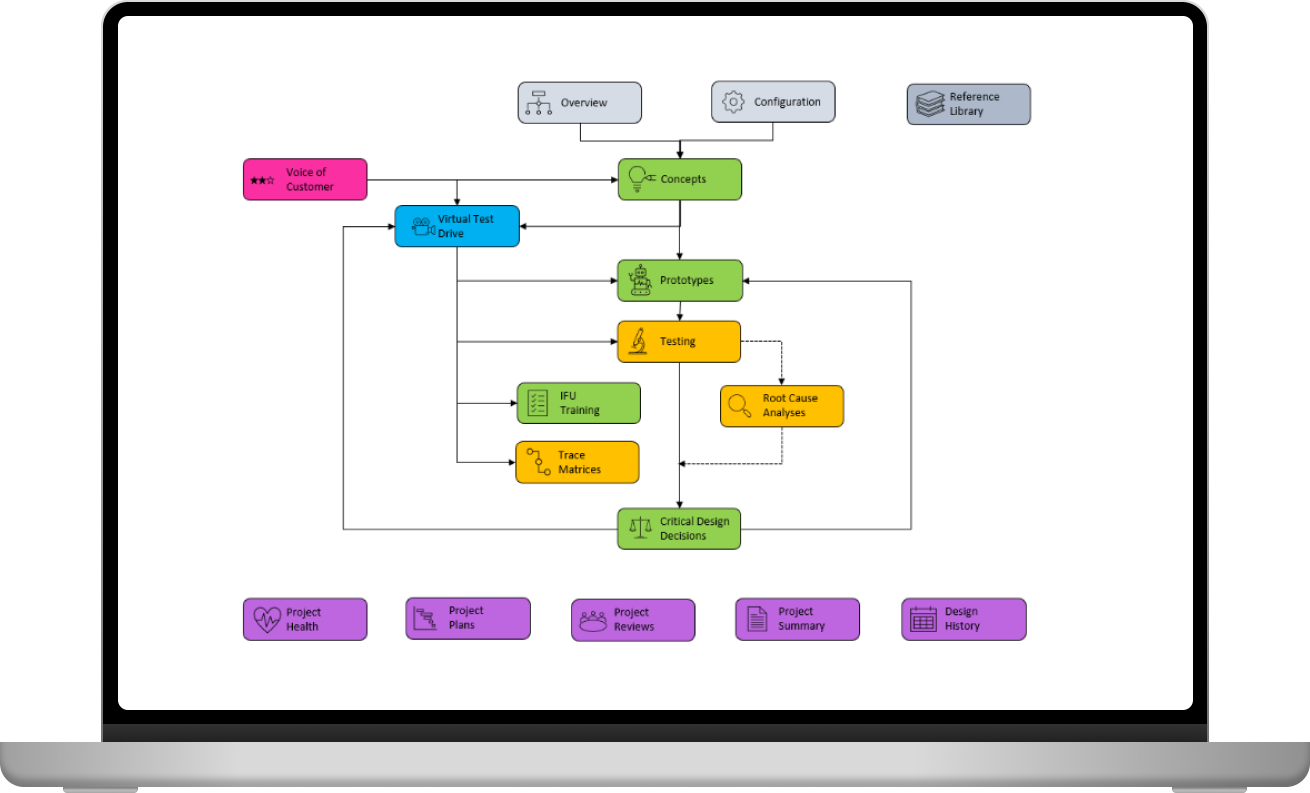
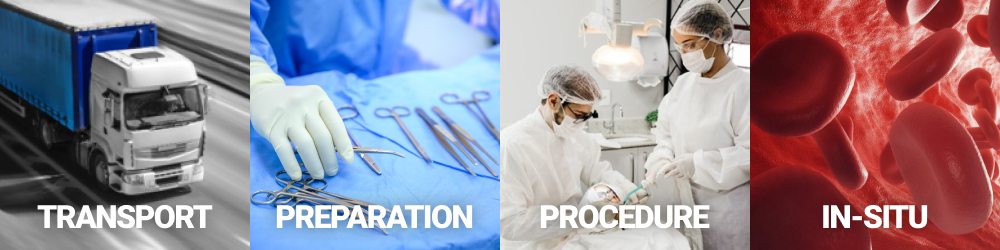

Take a virtual test drive through the entire use life of your product to identify user tasks, design requirements, critical operating conditions, safety hazards, and safety measures. CRISP converts the captured information into trace matrices.
CRISP issues professional reports and catalogues them chronologically. Your entire design history is just a mouse click away.
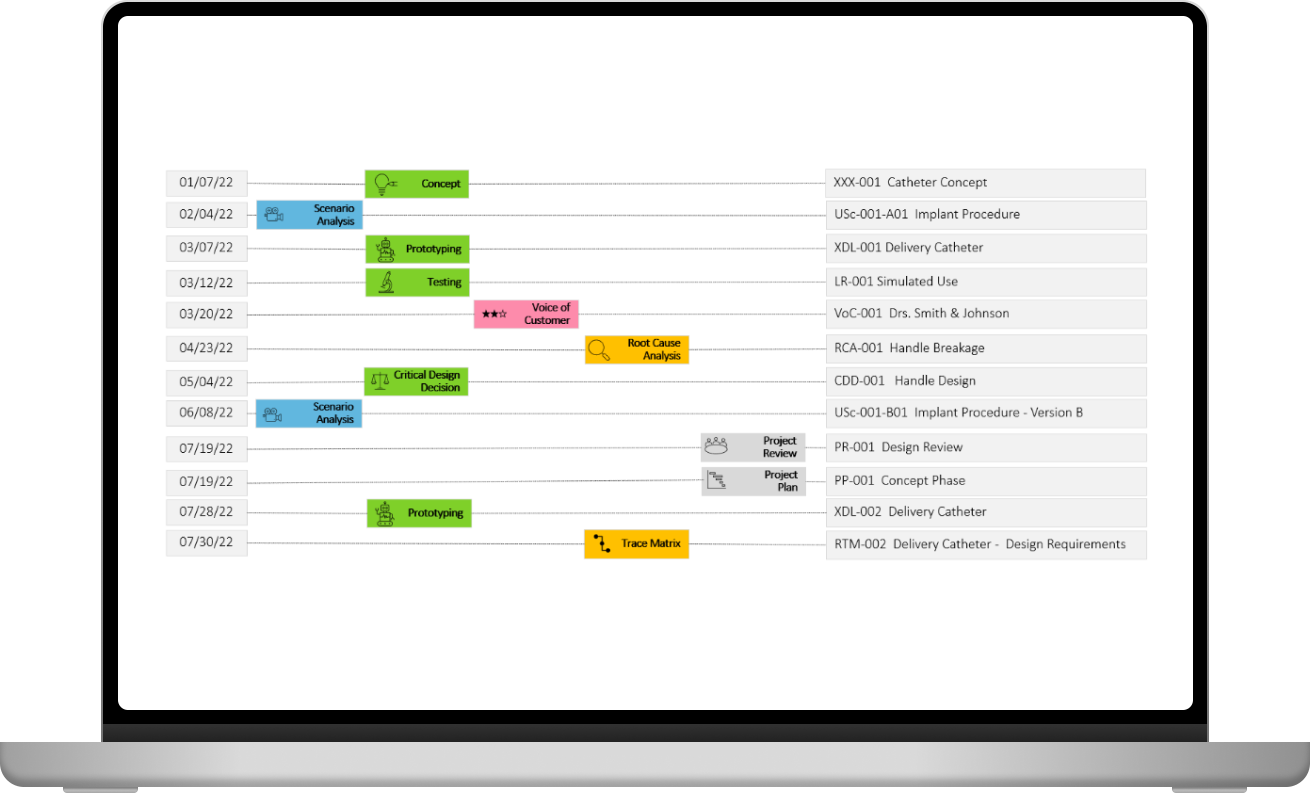
CRISP guides you through an in-depth performance and risk assessment.
Universal
CRISP can be configured to any type of medical product.
Comprehensive
CRISP helps to identify design requirements and hazards throughout the entire product use cycle.
CRISP helps you in selecting the best design solution.
Agile
CRISP supports the parallel evaluation of alternative design options.
Design-Centric
The CRISP health report identifies information gaps and design risks
CRISP supports the product development process all the way to first-in-human.
First-in-human
CRISP integrates the clinical protocol, product release, and investigator training into the performance and safety assessment.
Seemless
CRISP generates design requirement and hazard trace matrices for a seemless transition into QMS.


Request a DEMO and experience the unique features of CRISP
CRISP, the software for Continuous Risk and Performance assessment of medical devices. Patent pending technology developed by DualTrack, LLC.